Capsule Manufacturing Process
Capsule manufacturing involves several steps to ensure the production of high-quality capsules. Here is a general overview of the capsule manufacturing process:
-
Formulation Development:
Develop a formulation for the capsule content, which includes the active pharmaceutical ingredient (API) and any necessary excipients. The formulation should be optimized for stability, bioavailability, and patient acceptability.
-
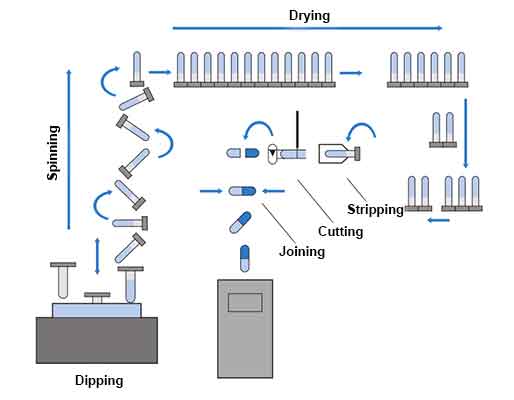
Preparing the Capsule Shell:
Capsules are typically made from gelatin or vegetarian-based materials. The capsule shells can be pre-made or produced on-site using capsule filling equipment. The shells are formed by dipping stainless steel pins into a gelatin solution and then drying the formed capsules.
-
Capsule Filling:
Capsule filling involves the placement of the desired amount of the formulation (API and excipients) into the capsule shell. This can be done manually or by using automatic capsule filling machines. The filling process should ensure accurate and consistent dosing.
-
Capsule Sealing:
After filling, the capsule shells need to be sealed to prevent leakage or tampering. The sealing process can be done using different methods, such as heat sealing or band sealing, depending on the type of capsules being manufactured.
-
Inspection and Quality Control:
Inspect the filled and sealed capsules for any defects, such as broken capsules, improper sealing, or inconsistent dosing. Quality control tests may include weight variation, content uniformity, dissolution testing, and other relevant tests to ensure the capsules meet quality standards.
-
Packaging:
Once the capsules pass inspection and quality control, they can be packaged into appropriate containers, such as blister packs, bottles, or sachets. The packaging should provide protection from moisture, light, and other environmental factors that may affect the stability of the capsules.