Validation Approaches for Water Systems
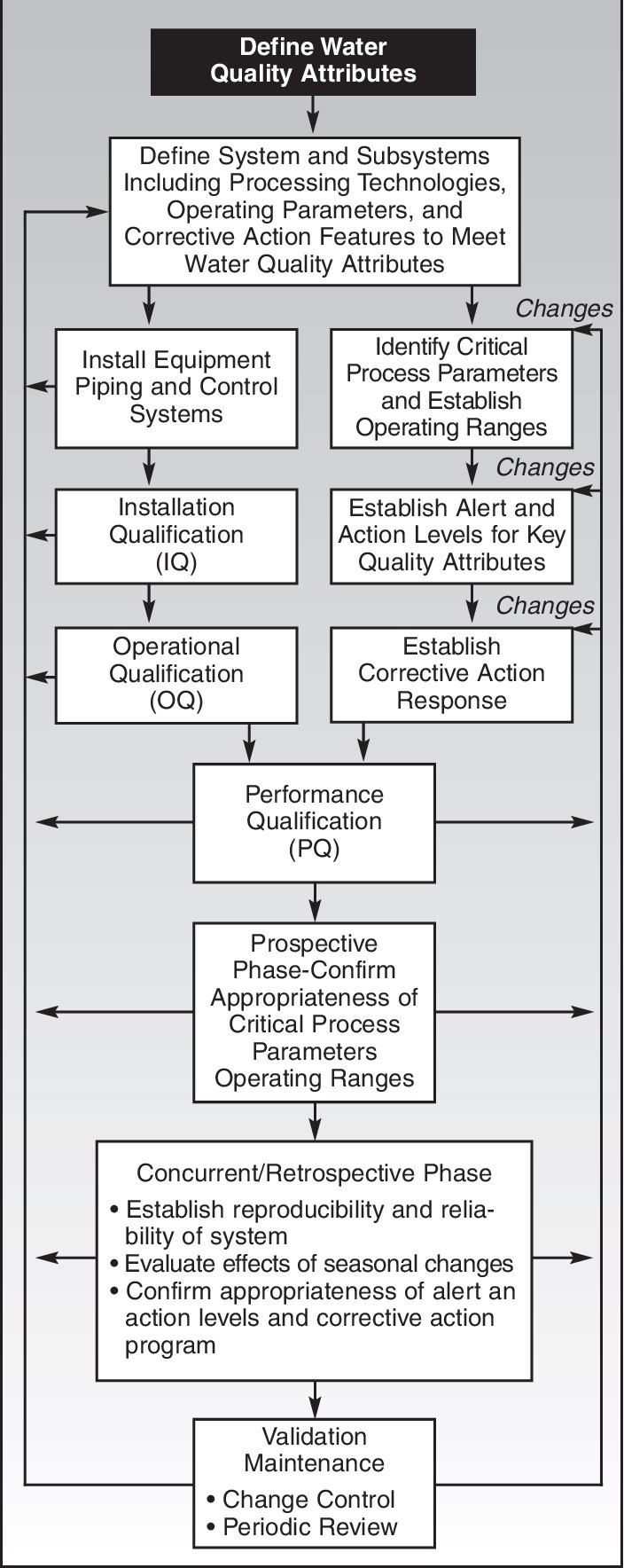
Purified Water (PW) System Validation:
- Design Qualification (DQ): This includes defining the system requirements, specifications, and intended use, as well as ensuring the design meets the regulatory and industry standards.
- Installation Qualification (IQ): Verifying that the PW system is installed correctly, including the equipment, components, and utilities.
- Operational Qualification (OQ): Testing and documenting the system's operational parameters and performance under normal operating conditions.
- Performance Qualification (PQ): Demonstrating that the PW system consistently meets the required specifications and can produce water of the desired quality.
Water for Injection (WFI) System Validation:
In addition to the steps mentioned for PW system validation, WFI system validation includes an additional focus on the generation and control of high-quality water meeting the specific requirements for WFI. The validation process typically involves detailed testing and documentation of the various stages, such as pretreatment, distillation, or other validated methods used for WFI production. The validation approach should include testing for critical parameters, such as conductivity, total organic carbon (TOC), bacterial endotoxins, and other relevant specifications outlined in the regulatory guidelines.
Sterile Water for Injection (SWFI) System Validation:
SWFI system validation encompasses the validation activities of both PW and WFI systems, with an additional emphasis on demonstrating and maintaining sterility throughout the distribution process. The validation approach should include validation of sterilization methods, such as steam sterilization or other validated methods used for achieving sterility. Validation activities should cover not only the generation of sterile water but also the storage, distribution, and handling processes to ensure the maintenance of sterility. Monitoring and control of critical parameters, such as microbial content, temperature, and pressure, are essential in SWFI system validation.
Considerations for IQ and OQ:
- Dead Legs: Dead legs are sections of piping that are not in continuous use and can create stagnant zones where microbial growth or accumulation of impurities may occur. It is important to minimize dead legs in the design to prevent such issues. If dead legs are present, they should be periodically flushed or subjected to a cleaning and sanitization procedure to maintain water quality.
- Temperature Control: The temperature of the distributed water should be considered to prevent microbial growth and ensure optimal conditions for use. Generally, the temperature should be maintained below the range that promotes microbial proliferation. The specific temperature requirements may vary based on the intended use and regulatory guidelines.
- Cooling Equipment: Cooling equipment, such as heat exchangers or chillers, may be installed in the water distribution system to lower the temperature of the water to the desired level.
- Temperature Monitoring: Temperature sensors or probes should be installed at appropriate locations within the system to monitor and control the temperature of the cooled water.
Process Qualification:
The duration of Process Qualification (PQ) for a water system depends on various factors, including the complexity of the system, the criticality of the water quality for the process, and regulatory requirements. The goal of the PQ is to demonstrate that the water system consistently produces water of the desired quality and meets the defined specifications.
The PQ for a water system typically involves running the system under normal operating conditions for an extended period of time, monitoring key parameters, and collecting data to evaluate the performance and consistency of the water quality. The duration of the PQ should be sufficient to capture variations in the water quality that may occur due to different operating conditions, start-up and shutdown procedures, and other relevant factors.
The exact duration of the PQ for a water system can vary depending on the specific requirements of the process and the regulatory guidelines applicable to your industry. It is important to consider factors such as the stability of the water quality, the frequency of water usage, and any seasonal variations that may impact the water system performance.